Mechanism of Action:
- Neuronox® is a Botulinum toxin type A injection acts by inhibiting the release of acetylcholine at the neuromuscular junction, leading to muscle relaxation.
Product Overview:
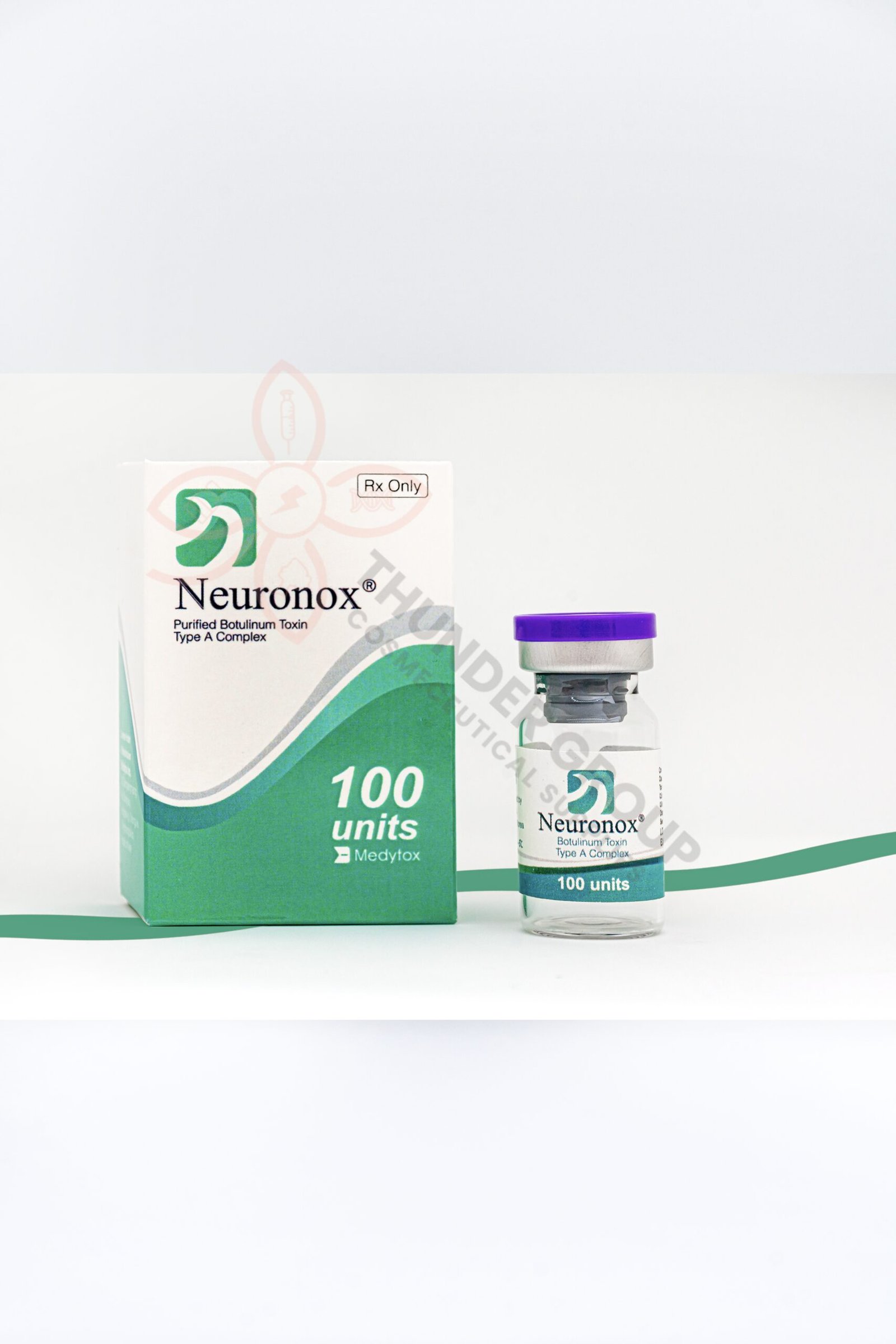
- Neuronox® is a botulinum toxin type A injection is Korea’s first and the fourth botulinum toxin type A product in the world, launched in 2006.
- Neuronox ® appears as a white vacuum-dried powder for injection in a colorless and transparent vial.
- Neuronox ® should become colorless transparent liquid when dissolved in the diluent (physiological saline solution).
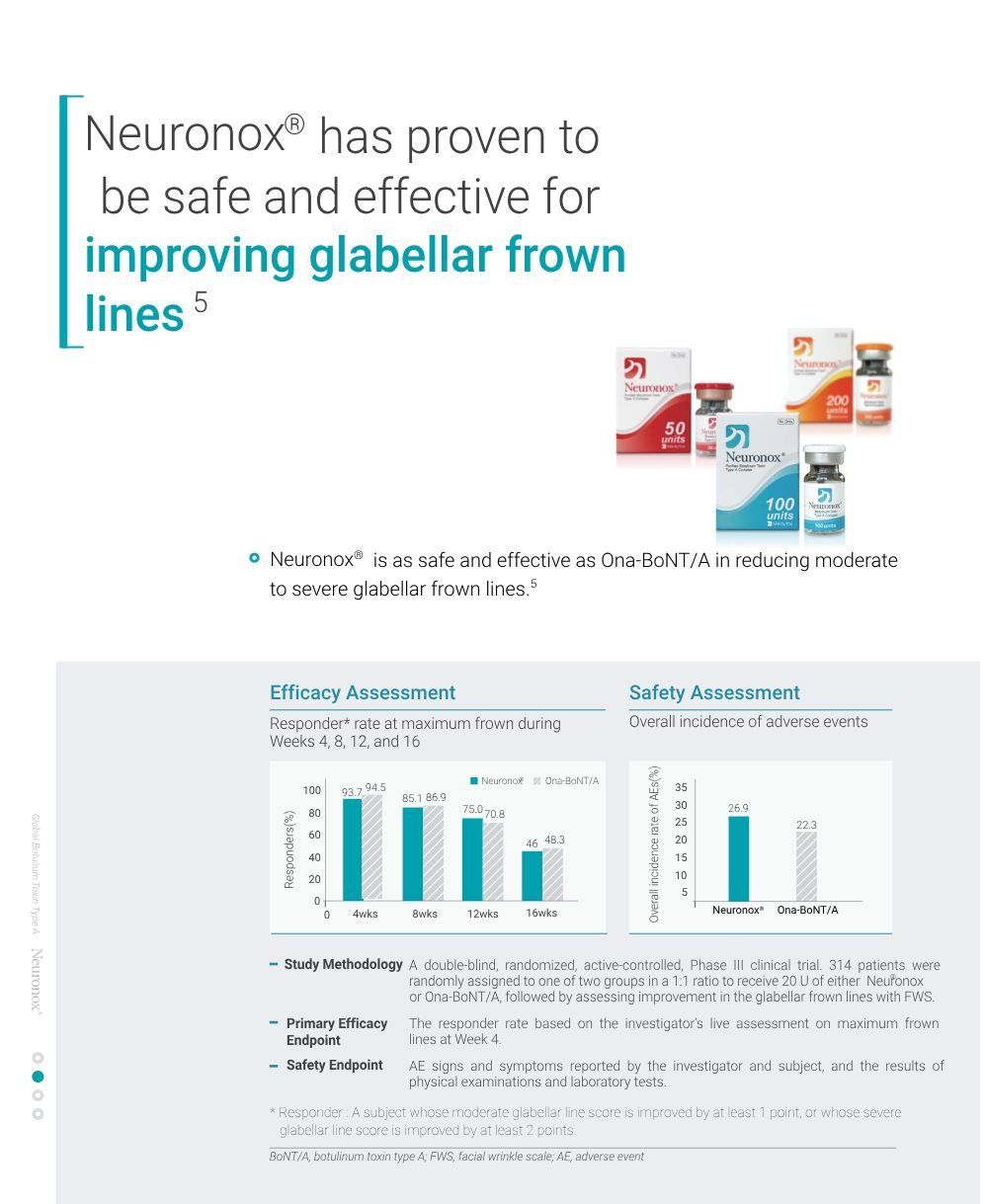
- Neuronox ® is a botulinum toxin type A drug used to treat muscle spasms in the neck, eye and foot, as well migraines, urinary incontinence, and excessive sweating.
- Neuronox ® is also used for cosmetic purposes to treat facial wrinkles by paralyzing muscles.
Advantages of Neuronox ® Botulinum Toxin Type A :
- Efficacy and Safety:
- C. botulinumtoxin A of Neuronox® has the same origins of strain as Allergan.
- Neuronox® has the Korean FDA (KFDA).
- Neuronox® has approvals in multiple countries, indicating compliance with stringent safety and efficacy standards.
- Clinical trials have shown Neuronox® to be highly effective in treating various neuromuscular conditions and cosmetic concerns, with results comparable to other leading brands.

2- Versatile Applications:
- Neuronox® is versatile and can be used for various indications, including reducing glabellar lines, crow’s feet.
- Also Neuronox® is approved for managing for the treatment of cervical dystonia in adults . AND blepharospasm in patients aged 12 years and older. blepharospasm . AND urinary incontinence in adults with neurogenic detrusor overactivity, such as in cases of spinal cord injury or multiple sclerosis.. AND in upper limbs spasticity for adults, which can result from conditions such as stroke, multiple sclerosis, cerebral palsy, or traumatic brain injury.
- Cost Effectiveness:
- Neuronox® is often more cost-effective compared to some other well-known brands, making it an attractive option for both patients and practitioners.
- Patient Satisfaction:
- High patient satisfaction rates due to effective outcomes and minimal side effects.
- Comparison with other brands:
- Neuronox® is often compared with other botulinum toxin products such as Botox (by Allergan), Dysport (by Ipsen), and Xeomin (by Merz Pharma), with differences in formulation, potency, and clinical applications.
Origin:
Made in Korea.