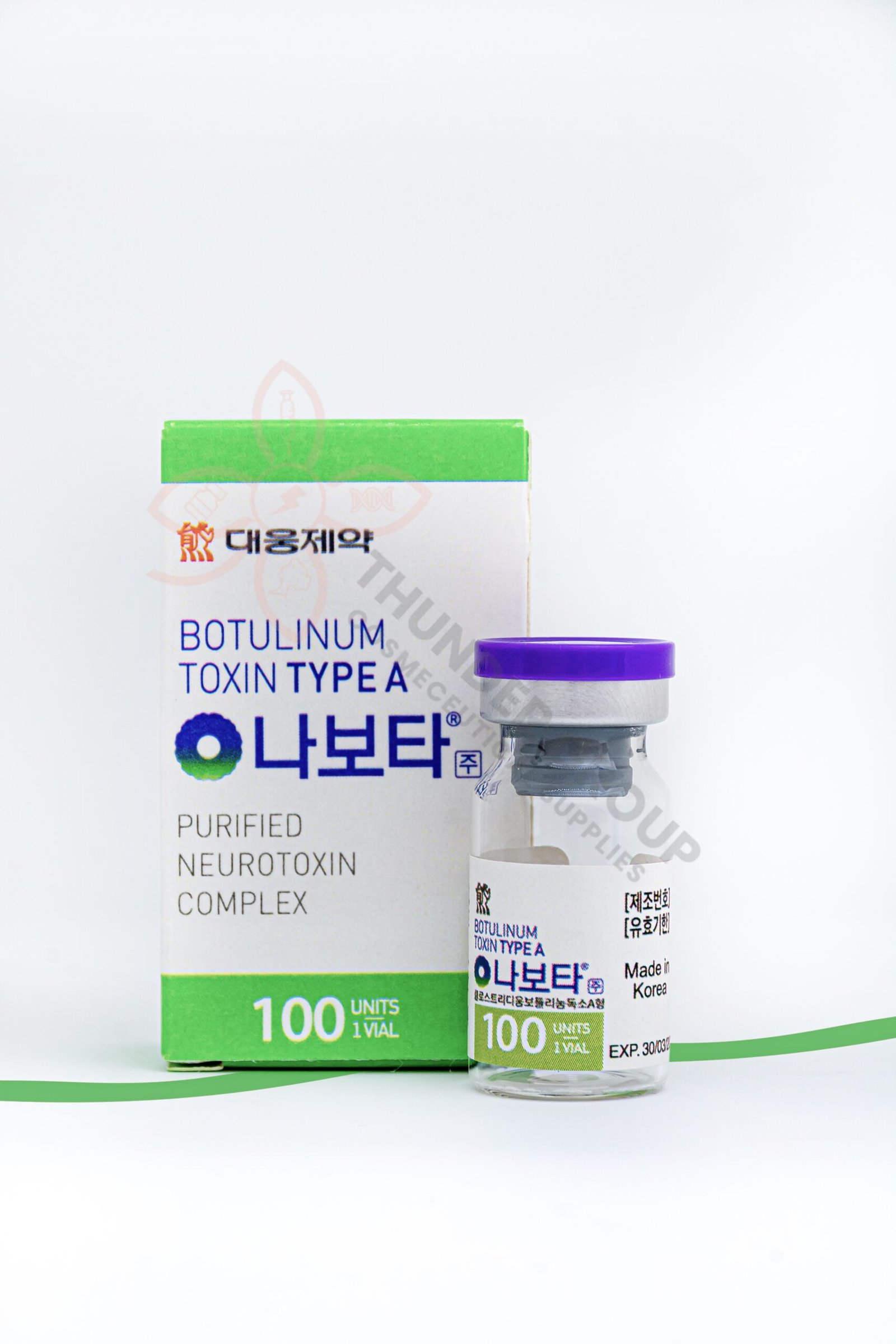
Nabota® Introduction:
- Nabota® is a purified form of botulinum toxin type A, produced from a toxin culture of the hall strain of Clostridium Botulinum grown in a medium containing trypticase and yeast extract.
- A series of purifying procedure were taken to obtain a crystalline complex. After re-dissolved and dialyzed the crystalline toxin, an accurate amount of the sterile filtered (0.2 microns) toxin were added to a solution containing gelatin-dextran-sucrose, then vacuumed.
Nabota® mechanism of action and how it works :
Nabota® is a protein made by a type of bacteria (Clostridium botulinum type A). It blocks the release of the presynaptic acetylcholine which causes muscle contraction. This temporarily weakens nerve activity in the muscles and helps reduce abnormal muscle contractions.
Product Overview:
- Nabota® Botulinum Toxin Type A appears as vacuum dried-freezed powder.
- Nabota® Botulinum Toxin Type A should become colorless transparent liquid when dissolved in the diluent (saline solution).
Nabota® Botulinum Toxin Type A used to treat both cosmetic and therapeutic indications:
– Cosmetic Indications as:
+ (Crow’s feet, Bunny Lines, Glabellar lines, forehead Lines, Smoking Lines and Platysma as well as chronic migraines, urinary incontinence, facial wrinkles and excessive sweating).
– Therapeutic Indications as:
+ Cervical Dystonia: Nabota® is approved for the treatment of cervical dystonia in adults, which involves abnormal head and neck posture due to involuntary muscle contractions; Check HereCheck Here
+ Blepharospasm: Nabota® is indicated for treating blepharospasm in patients aged 12 years and older, a condition characterized by involuntary tight closure of the eyelids; Check here.
+ Urinary Incontinence Due to Neurogenic Detrusor Overactivity: Nabota® is approved for treating urinary incontinence in adults with neurogenic detrusor overactivity, such as in cases of spinal cord injury or multiple sclerosis; Check here.
+ Upper Limb Spasticity: This approval includes the treatment of spasticity in the upper limbs for adults, which can result from conditions such as stroke, multiple sclerosis, cerebral palsy, or traumatic brain injury; Check here.
Nabota® (100 iu) important dilution technique hint:
- For Facial Wrinkles treatment cases: Nabota® (100 iu) must be diluted by 2 ml saline.
- Retouching MUST BE after 10-15 days, in case the client needs fine retouches.
For Baby Botox or Meso Botox:
- After Nabota® dilution; Take 10 international unit toxins (iu) OR 0.1 CC from the diluted Nabota® vial into an insulin syringe.
2. Then, add 0.4 CC saline in the same insulin syringe mentioned above.
3. Now, Baby Botox or Meso Botox is ready for action by INTRADERMAL INJECTION.
Advantages of Nabota® Botulinum Toxin Type A :
- Nabota® FDA approval, also known as Jeuveau in the USA, received the FDA on February 1, 2019, as meets the high quality standards. You can view the official FDA approval document here
2. Cost Effective: Nabota® has reasonable price compared to others.
3. Volumes: As Nabota® is available in 50 iu and 100 iu.
4 . Preserves Facial Expressions: Unlike some alternatives, Nabota® maintains natural facial expression avoiding the “frozen look often associated with wrinkle treatments.
5. Quick Action: Nabota® delivers rapid results and effectively minimizing the appearance of wrinkles in a short span of time.
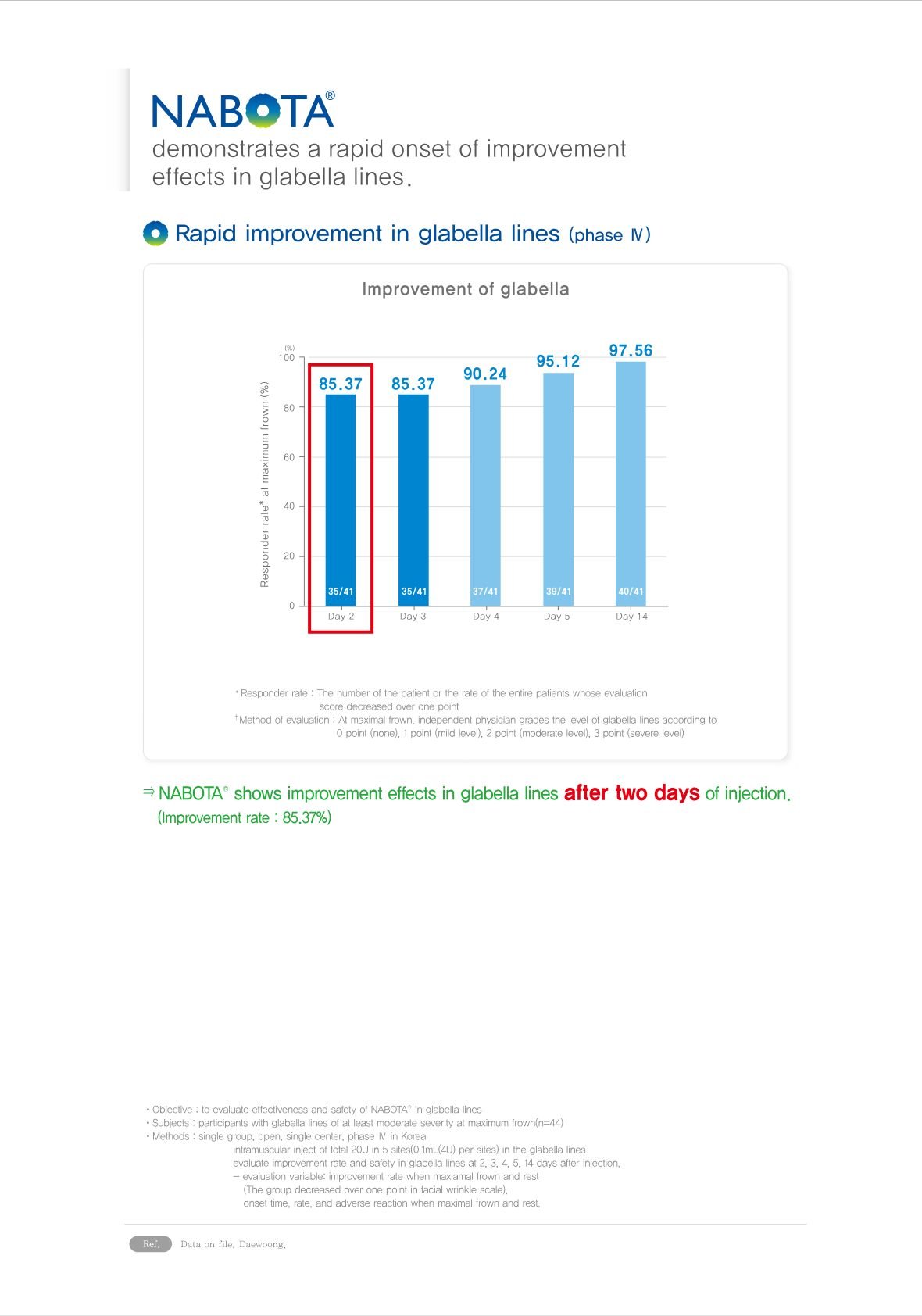
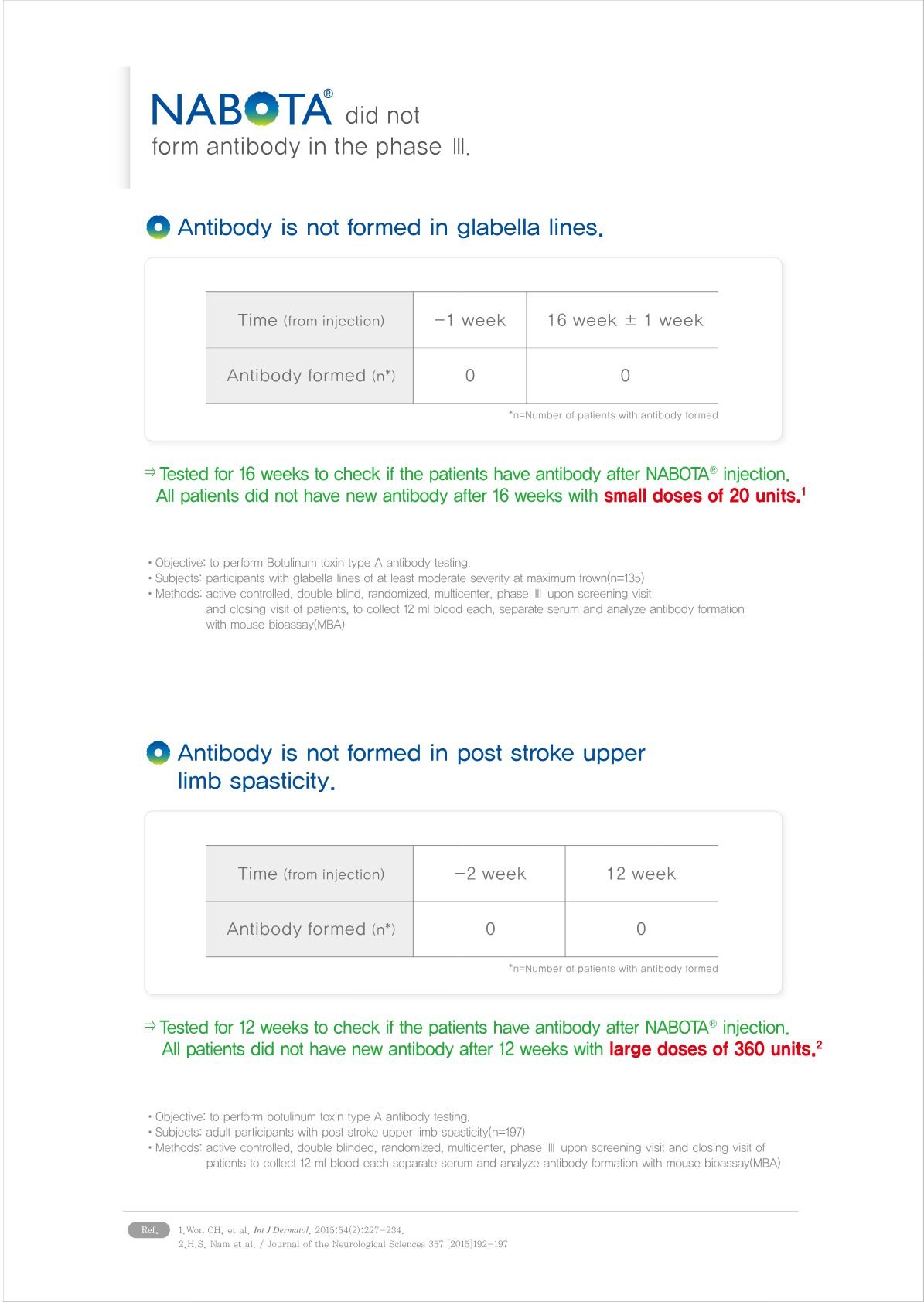
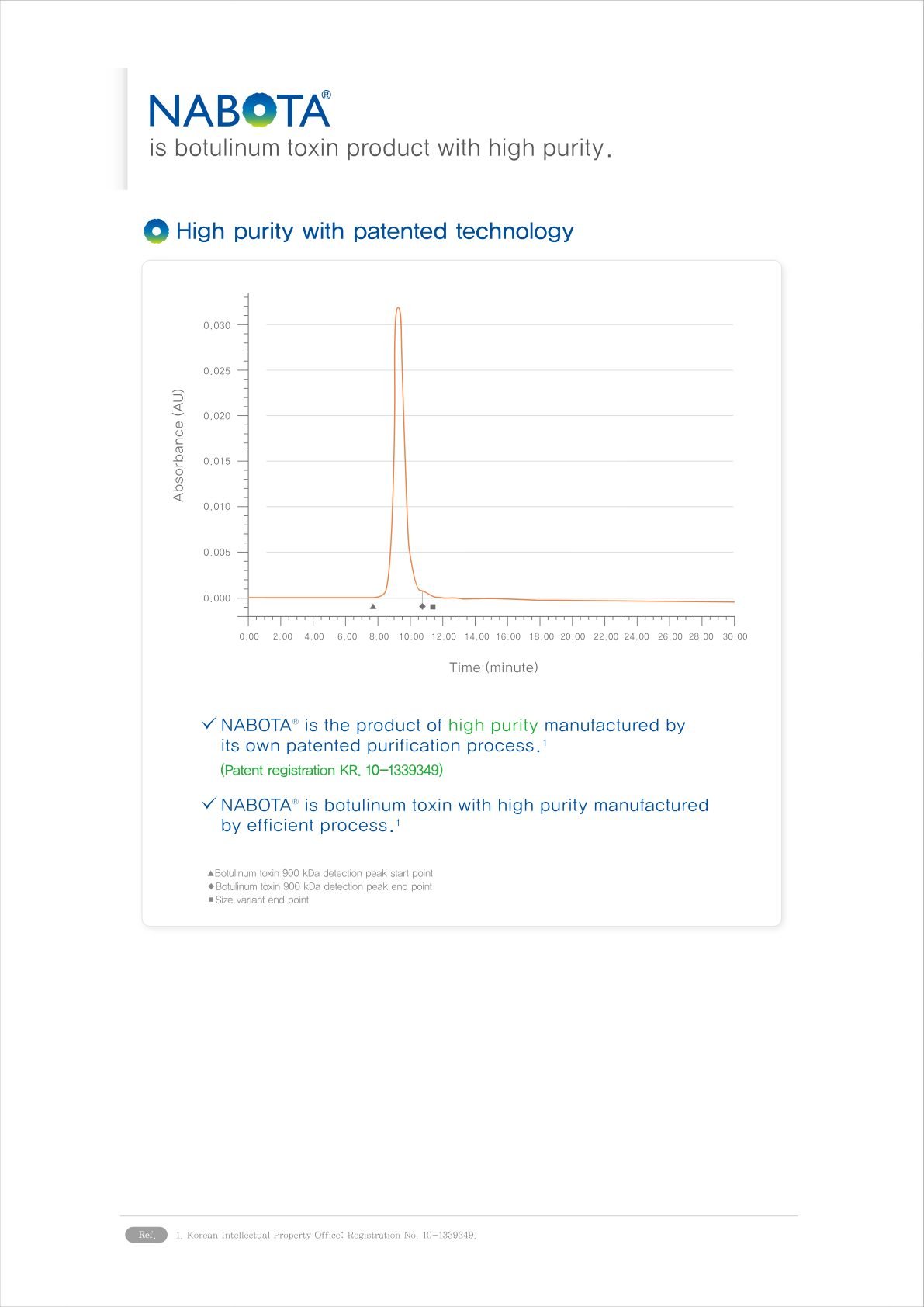
6. High Purity relevant to antibodies decline: Nabota®’s formulation is designed to be highly pure and consistent, which helps in reducing the risk of antibody development. The absence or reduction of complexing proteins in its formulation plays a crucial role in its low immunogenicity.
Origin:
Made in Korea.